Abstract
Introduction:
Immunotherapies like Inotuzumab ozogamicin (InO), Blinatumomab and Chimeric antigen receptor T cell therapy (CAR-T) have revolutionized outcomes in patients with Relapse/Refractory Acute Lymphoblastic Leukaemia (R/R ALL) with event free survival (EFS) of 50% at 1 year (Maude et al. 2018).
Optimal phasing of these agents has not been clearly defined and depends on antigen expression on blasts, CNS status, T cell number and prior disease response. InO, a CD22 targeting antibody-drug conjugate has proven efficacy in adults (Kantarjian et al., 2016) and children as a bridge to allogeneic stem cell transplantation (allo-SCT) with a favourable toxicity profile (Brivio et al. 2021). However, outcomes following the use of InO prior to CAR-T therapy are still not established.
This study provides a retrospective analysis of children and young adults who received InO as part of pre-CAR-T management (before leucapheresis or as bridging therapy to CAR-T infusion).
Methods:
Patients aged 0-25years with R/R ALL were eligible for the study if they received InO pre-CAR-T therapy. Retrospective data collection was performed using a standardised form from CAR-T centres in the UK. Response to CAR-T therapy and/or relapse was evaluated at 1, 3, 6, 12 months or at last follow-up. Results were compared to a control cohort of R/R ALL patients treated with tisagenlecleucel but without preceding InO, over a contemporaneous period, at the largest paediatric CAR-T centre in the UK.
Results:
Fourteen patients from 5 paediatric and young adult centres in the UK received InO after screening for CAR-T therapy. InO was used pre-leucapheresis, as bridging and for both in 2, 11 and 1 respectively. Two were excluded from outcome analysis (1 adolescent with Trisomy 21 and transfusion induced hepatic siderosis died due to VOD while awaiting CAR-T production and 1 failed CAR-T manufacture despite achieving an adequate T cell harvest as per manufacturer guidance). Twelve (85%) patients were able to receive CAR-T infusion at a median of 1 month (range 0.7-5.6 months) from first InO dose. InO was well tolerated with 21% developing febrile neutropenia and grade 4 cytopenias. Use of InO pre-leucapheresis led to successful manufacture of CAR-T cells in 2/3 (66%). InO group was compared with 27 children who received CAR-T without preceding InO over the same time period. Table 1 summarises patient and CAR-T characteristics of the two groups which were comparable. Median follow-up of the InO and non-InO groups was 10 months (range 2.8- 30.1 months).
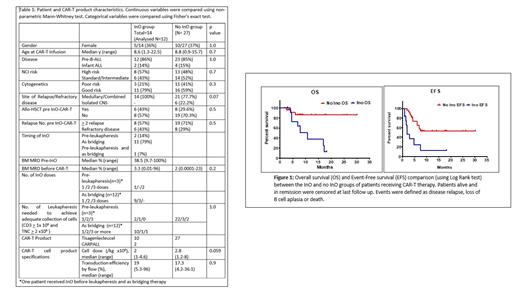
In the InO group(n=12), 3 (25%) remained leukemia free at last follow up. Nine patients (75%) relapsed. All relapses occurred within 6 months from CAR-T infusion. Seven (58%) of those who relapsed died at a median of 7.8 months post CAR-T infusion and 2 (16.6%) were salvaged with further therapy. Subsequent therapy (alternate CAR-T and/or allo-SCT) was carried out in 4/12 (33%) patients in the InO group and in 5/27 (18.5%) in the non-InO group (p= 0.2). EFS was significantly higher in the non-InO group (53% vs 12%, p=0.0009), as was OS (86% vs 13%, p=0.004) (Figure 1).
Conclusion:
This study provides a direct comparison between two contemporaneously treated cohorts with R/R ALL receiving CAR-T therapy. InO prior to CAR T cell therapy was well tolerated despite the cohort being heavily pre-treated except for one patient who developed fatal VOD prior to CAR T cell infusion. InO was given for disease debulking, rather than to achieve MRD negativity and hence majority of the cohort (75%) received only 1 dose of InO in bridging rather than the usual schedule of three doses weekly per cycle.
Outcomes for the non-InO group were comparable to those treated on the ELIANA study but the OS of the InO group was significantly lower (13% vs 86% p= 0.004). Potential reasons include an impact of InO on function of CAR-T cells in vivo or a deleterious effect of InO on the B cell compartment such that CAR-T cells prove less effective. Due to the retrospective nature of the study, it is possible there was an inherent bias to use InO in patients with more resistant disease, although the very comparable pre CAR-T bone marrow disease burden in each group suggests this was not the case. The small size of the cohorts may also have exaggerated differences in outcome.
Ultimately, randomised controlled studies of InO pre-CAR-T are required before firm conclusions about its impact on outcomes post CAR-T therapy can be ascertained.
Sharplin: Kite Gilead: Honoraria; Novartis: Other: Travel Support. Nicholson: BMS/Celgene: Consultancy; Kite, a Gilead Company: Other: Conference fees, Speakers Bureau; Novartis: Consultancy, Other: Conference fees; Pfizer: Consultancy. Amrolia: ADC Therapeutics: Other: Named inventor on a patent which is being transferred to ADCT.; Autolus: Patents & Royalties. Ghorashian: Novartis: Honoraria; UCLB: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal